Page 64 - Combine
P. 64
International Journal of Trend in Scientific Research and Development (IJTSRD) @ www.ijtsrd.com eISSN: 2456-6470
These types of bonds have a light terminal hydrogen atom.
Oscillations of this kind of bonds in the molecule are
experienced only by a slight effect from the rest of the
molecule. It can be argued that a set of energetically unequal
hydrogen bonds is realized in such compounds [26,27].
Strong vibrations at 1610 cm-1 confirmed the presence of
the carbonyl group of the carboxymethyl anion (-C = O). The
absence of a wide band in the region of 2500-2800 cm-1 and
the high value of the position of the stretching vibration band
of the carboxyl group indicate that the carboxyl groups are
not at a high level, and they do not form hydrogen bonds
with each other. Absorption bands of about 1423 and 1325
cm-1 belong to the —CH2 group and the hydroxyl group (—
OH), respectively. Absorption in the region of 1140-1065 cm-
1 refers to vibrations of CO groups. With an increase DS of
CMC SC, the intensity of the bands decreases, characterizing
the planar deformation vibrations of hydroxyl groups. A
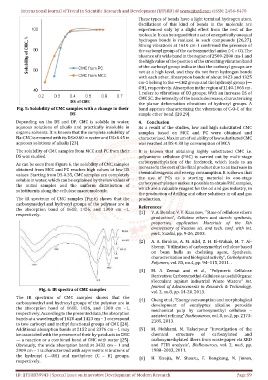
Fig. 5: Solubility of CMC samples with a change in their band appears characterizing the vibrations of C-O-C of the
DS simple ether bond. [28.29].
Depending on the DS and DP, CMC is soluble in water, 4. Conclusion
aqueous solutions of alkalis and practically insoluble in As a result of the studies, low and high substituted CMC
organic solvents. It is known that the complete solubility of samples based on MCC and PC were obtained and
Na-CMC is ensured with its DS≥0.60 in water and DS≥0.45 in characterized. Maximum of solubility of low substituted CMC
aqueous solutions of alkalis [23]. was reached at DS-0.38 by consumption of MCA
The solubility of CMC samples from MCC and PC from their It is known that obtaining highly substituted CMC i.e.
DS was studied. polyanionic cellulose (PAC) is carried out by multi-stage
carboxymethylation of the feedstock, which leads to an
As can be seen from Figure 6, the solubility of CMC samples
obtained from MCC and PC reaches high values at low DS increase in the cost of the final product due to an increase in
values. Starting from DS-0.35, CMC samples are completely chemical reagents and energy consumption. It is shown that
soluble in water, which can be explained by the low values of the use of PCs as a starting material in one-stage
the initial samples and the uniform distribution of carboxymethylation makes it possible to obtain PAC samples,
which are a valuable reagent for the oil and gas industry, in
substituents along the cellulose macromolecule.
the production of drilling and other solutions in oil and gas
The IR spectrum of CMC samples (Fig.6) shows that the production.
carboxymethyl and hydroxyl groups of the polymer are in
the absorption band of 1618, 1426, and 1300 cm –1, References
respectively. [1] V. A. Bondar, V. V. Kazansev, “State of cellulose ethers
production”, Cellulose ethers and starch: synthesis,
properties, application. Materials of the Xth
anniversary of Russian sci. and tech. conf. with int.
part., Suzdal, pp. 9-26, 2003.
[2] A. A. Ibrahim, A. M. Adel, Z. H. El-Wahab, M. T. Al-
Shemy, “Utilization of carboxymethyl cellulose based
on bean hulls as chelating agent, Synthesis,
characterization and biological activity”, Carbohydrate
Polymers, vol. 83, no.4, pp. 94–115, 2011. .
[3] M. A. Zeenat and et al., “Polymeric Cellulose
Derivative: Carboxymethyl-Cellulose as useful Organic
Flocculant against Industrial Waste Waters” Int.
Journal of Advancements in Research & Technology,
Fig. 6: IR spectra of CMC samples
vol. 2, no.8, pp. 14-20, 2013.
The IR spectrum of CMC samples shows that the [4] Cheng et al., “Energy consumption and morphological
carboxymethyl and hydroxyl groups of the polymer are in development of eucalyptus alkaline peroxide
the absorption band of 1618, 1426, and 1300 cm –1, mechanical pulp by carboxymethyl cellulose –
respectively. According to the presented data, the absorption assisted refining” BioResources, vol. 8, no.2, pp. 2173-
bands at a wavelength of 1620 and 1423 cm – 1 correspond 2185, 2013.
to two carboxyl and methyl functional groups of CMC [24].
Additional absorption bands at 2152 and 2376 cm – 1 may [5] M. Mohkami, M. Talaeipour “Investigation of the
be associated with the presence of their by-products in CMC chemical structure of carboxylated and
— a reaction or a combined bond of CMC with water [25]. carboxymethylated fibers from waste paper via XRD
Obviously, the wide absorption band at 3432 cm – 1 and and FTIR analysis”, BioResources, vol. 2, no.6, pp.
2909 cm – 1 is characterized with asymmetric vibrations of 1988–2003, 2011.
the hydroxyl (—OH) and methylene (C – H) groups, [6] H. Xiaojia, W. Shaozu, F. Dongkang, N. Jinren,
respectively.
ID: IJTSRD39943 | Special Issue on Innovative Development of Modern Research Page 59